Many researchers are looking for ways to improve desalination and other freshwater tech to help us solve the freshwater crisis, like what we’re seeing in the southwest US and other parts of the world. There’s some promising advances in both efficiency and quality compared to what’s currently available. With these new technologies we can pluck the water from the air at the push of a metaphorical button, so are they finally the answer we’re looking for? Let’s see if we can come to a decision on this.
Fresh water is THE great equalizer: no matter who you are or what your background is, water is crucial to our survival and way of life. While many of us have this fundamental resource literally “on tap”, not all of us are so lucky, and more of us may face that same dilemma in the near future. Just take a look at the worsening drought in the southwest US, where some areas are seeing some of the worst dry spells they’ve had in the past 1,200 years1. Record heat, major soil moisture loss, and historically low reservoirs are only some of the symptoms that are likely to stick around.
It’s not just the US, either. The World Health Organization reports that 785 million people lack even basic drinking-water services, and by 2025, half of the world’s population will be living in water-stressed areas or conditions2, and it’s predicted that ¾ of the world’s population will suffer from freshwater shortages by 2050.3 Even though water covers 70% of the Earth’s surface, only 2.7% of that is freshwater, and only 0.3% can be directly used by humans as-is3. This is going to be a problem close to home.
So we have two options: either stretch the water we already have, or find it somewhere else (preferably in that 97% worth of water previously unavailable to us).
We already have some idea of how to do this via techniques like water harvesting (collecting rainwater runoff)4 and desalination (removing dissolved salts from otherwise undrinkable water to make it suitable for human consumption)2. Desalination has been a huge target, especially for coastal and/or rural communities who are tempted to use the giant wealth of water that’s already practically in their backyards.
Those desalination technologies are generally one of three types: thermal (aka distillation), membrane (like reverse osmosis) and charge-based (which use ion exchange processes). Thermal tends to be good for desalinating a lot of water at once, to the tune of several thousand cubic meters a day (which makes it appealing at industrial levels). Charge-based desalination is better for brackish water sources along with small-scale systems (more along the lines of a few hundred cubic meters a day). And membrane technologies, perhaps the most well known of the bunch, can be optimized for practically any production level, making it one of the most commercially utilized desalination technologies on the market.2
So why aren’t there desalination plants everywhere? Well, we’re still trying to find the right flow. For one, desalination is an energy-intensive process, which can make it costly in areas not flush with energy funding (like Saudi Arabia). You also have to consider the environmental impacts from brine runoff (aka the residual water that holds all that extra salt and other gnarly stuff after the desalination is said and done). On top of all of that, the technological limits of the desalination tech we have currently all seem to have made desalination seem nearly dead in the water–at least, at first glance.
There are quite a few researchers determined to change this. Among the many projects underway, two in particular have made some exciting headlines–and have interesting results to back them up. While they’re just two of many, these could be the building blocks of what’s to come. While they may not be the solution to solving large scale water needs, they do give us an indication of the direction for fresh water technology, especially for more targeted applications. One of the advances gets freshwater at the push of a button WITHOUT filters, and the other can pull water out of the air without a power source, even in the middle of a desert. But are they worth the hype, and what role can they play in the freshwater crisis? Let’s take a deeper look.
MIT “Suitcase”
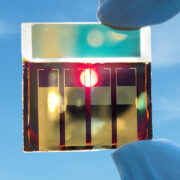
First, from our friends at MIT, we have a new, lightweight and portable desalination unit that can generate clear, clean drinking water without the need for filters or high-pressure pumps. The device itself is about the size of a suitcase, weighs less than 22 pounds, and requires less power to operate than a cell phone charger5.
Most commercially available portable desalination units rely on high pressure pumps to push the salty water through the filters, which not only makes it hard to make into a compact device, but it also requires extra maintenance and energy to keep everything running smoothly. MIT’s device, on the other hand, uses a two-step setup including ion concentration polarization (or ICP, for short).
ICP applies an electrical field to membranes placed above and below a channel of water. (think of these like electrified bumpers on a bowling lane). These membranes then repel positive or negatively-charged particles (like salt, bacteria, and viruses) as they flow past. These charged particles are then funneled into a second stream of water which is discharged at the end of the process. ICP doesn’t usually remove ALL of the salts flowing in the middle of the channel, so the researchers added a round of electrodialysis to remove those stubborn residual salt ions. Researchers found the optimal setup to include a two-step ICP process where water flows through six modules in the first stages, three modules in the second stage, and is followed by a single electrodialysis process to finish out. This setup minimized the energy usage required while also making sure that the process was self-cleaning.
This means this device falls squarely under the “charge-based” desalination techniques2 . Most rely on filters and other membrane techniques, which not only require strong pumps to operate, but also are prone to fouling and buildup on the pores by the salts and contaminants it’s meant to remove.6 The more contamination in the filter, the harder the pump has to work. As a contrast, in MIT’s device, the water never actually flows through the electrified membranes, which means you don’t need to worry about pressure OR particulates–basically a win-win. Even if particles get trapped, researchers can reverse the polarity of the electric field and electrostatically “sweep” those particles off the membrane easily5. The best part … the device can be driven by a small portable solar panel, and at the end of the day, the estimated cost of the unit is in the ballpark of around $50.
Low-cost water harvesting gel
What about the communities who aren’t close to the shoreline–are they out of luck? Not if the scientists at the University of Texas at Austin have something to say about it. Researchers have developed a low-cost gel film (all made from abundant materials) that can pull water from the air, even in drier climates.
Let’s be clear: the idea behind this isn’t really new. In terms of “pulling drinking water out of thin air”, it’s a pretty old trick. In fact, hydropanels have already beaten this gel to the market. Crudely put, they’re basically a dehumidifier strapped to the back of a solar panel. More specifically, these panels use solar power to convert water vapor in the air into a liquid state, collecting it into a reservoir where the water is mineralized for consumption7. It’s really straightforward. As I said, it’s basically a dehumidifier.
This water harvesting gel aims to do pretty much the same thing, with one major change: there’s no electricity required. A single kilogram of this gel can generate more than six liters of water per day in areas with less than 15% relative humidity, and up to 13 liters in areas with up to 30% relative humidity8. That makes it especially useful in drier areas like the southwest US, and the research was funded by the US Department of Defense’s Defense Advanced Research Project Agency (DARPA) in part to find ways to provide drinking water for soldiers in arid climates.
It’s made from a mix including renewable cellulose and konjac gum, whose open pore structure and hydrophilic nature (at room temperature) speeds up the moisture capture process. The thermo-responsive cellulose is also hydrophobic when heated, which helps to release the collected water immediately while also minimizing the amount of energy you need to keep the reaction going9.
It’s a relatively simple reaction, which researchers believe to be one of this gel’s biggest strengths. Making more of the gel is fairly simple too: all you need to do is mix the precursor, let it set for two minutes, then freeze dry. You can peel the gel off and use it immediately after that, and the gel itself is flexible and moldable, so you can make it in a variety of shapes and sizes. The researchers ultimately envisioned this as something that people could buy at your local hardware store and use in their own homes–even in the desert!8
For sure, these two projects–while extremely cool–aren’t exactly breaking the status quo by themselves. However, they do represent some interesting developments going on in the industry at large. So let’s explore that a bit: are they too good to be true?
Assessing the Suitcase
First, let’s take a closer look at MIT’s portable desalination device. Keep in mind, this project is one that has been in the making for over 12 years. Now that an official prototype has been tested, we need to ask: can we finally get efficient, effective desalination at the push of a button? How excited should we be about this?
Well, there’s one thing to be excited about: we have an actual field test, not just a small-scale experiment in the confines of the lab. Researchers Yoon and Kwon field-tested the device at Boston’s Carson Beach, literally putting the feed tube in the water and getting a cup of clean, drinkable water in a matter of thirty minutes. Not only that, but that cup of water exceeded World Health Organization quality guidelines while cutting the amount of suspended solids by a factor of at least 10. Not bad!
Of course, the device needs to generate more than one cup of water at a time: the prototype generates drinking water at a rate of about 0.3 liters per hour, and it only requires 20 watts of power per liter5 2. According to the U.S. National Academies of Sciences, Engineering, and Medicine, a man living in a temperate region would need about 3.7 liters of water per day.10 That means the prototype would need about 12 hours to collect enough water.
This device bucks the trend of using reverse osmosis for portable desalination devices, which actually makes sense in a lot of ways. Charge-based desalination techniques are more suited for brackish water feeds, and they also tend to have higher water recovery rates (85-90%) than reverse osmosis does (25-80%)2. Since we don’t want a drop to go to waste, that adds up fast. As an added bonus, the system would also remove many contaminants, viruses and bacteria at the same time.6
Reverse osmosis is usually considered the most promising option for smaller systems and places like islands and coastal communities, so the fact that ICP is challenging this status quo is a pretty big deal. Converting seawater to potable water usually takes a lot of electrically-powered pumps, well-maintained filters, and access to a reliable power source–something that’s not in huge supply in places like coastal communities and disaster areas.
We’re often impatient to turn small-scale energy advances to a larger scale, but in this case, the device’s small-scale nature is actually one of its biggest strengths. The device’s portability and low-maintenance design makes it perfect to deploy in remote and resource-limited areas, such as rural communities, cargo ships, or places hit by natural-disasters. You could use it day-to-day in a village.6. Factor in the low expected cost of this device–about $50 each–and you could potentially deploy a BUNCH of them at a time in those areas in need, once they’re commercially available.
We do still have to ask, though: what environmental impacts would this have? While small, this device is still shadowed by desalination’s unfortunate reputation in this regard. Brine discharge (aka the discharge with high levels of salt products) is a common problem, and a lot of energy is usually needed to achieve a near-zero liquid discharge (like thermal desalination methods). You can add extra steps to mitigate the brine problem, but as the desalination method gets more complicated, it also gets more costly, and you introduce more room for error.2
ICP device’s second deionization process seems to combat this problem, but we might need more info on how to handle potentially damaging discharge. (Granted, we likely wouldn’t see the large-scale damage done by major desalination plants, but that shouldn’t give us license to ignore it completely). Does this device remove non-charged pollutants that don’t have an electric charge, like industrial pollutants? Earlier iterations of the project indicate that it doesn’t. The final product may require at the LEAST a charcoal filter to cover its bases there6.
There’s still some room to grow, and the researchers are eager to keep up that momentum. They’re hoping to make the device more user-friendly, scalable, and energy efficient in the future, and they’re hoping to do so via a startup that researcher Han plans to launch in order to commercialize the technology. (For renewable-tech fans that bemoan how these advances tend to get stuck in the lab, having a push towards the market is a welcome change).
Assessing the Gel
As for the University of Pennsylvania’s water harvesting gel: did we mention you can use this in the DESERT? Sorry, not trying to drive that point into the sand; but it’s just a really cool feature, and one that makes this gel especially effective in the areas hit hardest by recent droughts.
Like the MIT device, this gel also seems to be a small-scale solution, possibly to supplement households or small businesses. Its 6-liter yield is only the start, according to the researchers. You could use thicker films or absorbent beds to optimize and increase the amount of freshwater they produce, and since the film itself is flexible and moldable, the application possibilities are practically endless.
This certainly isn’t the only tech out there that pulls water out of thin air, but those other solutions tend to be energy-intensive, and they don’t produce much either. The fact that this gel works without an energy source is huge in that regard. (You can speed up the reaction thermally, but it’s not required; you can practically “set it and forget it”.)
How about the environmental impact? That’s something we’d have to study in more detail. The ingredients themselves are reported to be nontoxic, and unlike desalination techniques, you’re not going to get that sickly salty brine that causes havoc once it’s discharged. Many of the ingredients are biodegradable (like cellulose and konjac gum), so once the gel mold exhausts its lifespan, there could be some promising environmentally-friendly disposal options.
Of course, that brings up another big unknown at this point: what is this gel’s lifespan? Unfortunately, that’s not something that’s been reported yet. That will make a huge difference in how easily this technology can be scaled up, because if you’re constantly needing to replace a mold that has lost its vitality, that could make this “easy” solution just as cumbersome as its hydropanel counterparts.
While the researchers envisioned this gel as something that people could someday buy at a local hardware store and use in their own homes, there is some scalability to explore here. The gel’s simple reaction makes it straightforward to use (which means less time tinkering with filters, mechanical parts, and other complicated maintenance items that could hamper larger-scale applications). The low cost is also a huge bonus. The materials themselves cost a measly $2 per kilogram. Compare that to the rising costs of fuel and electricity, and that makes this low-energy alternative pretty appealing!
Is It Enough?
You may be wondering: is this enough?
Unlike other exciting advances – like fluorinated nanotubes for desalination membranes, wick-free solar set-ups, and a low-energy battery electrode deionization system from the University of Pennsylvania – the two technologies we’ve talked about here actually have prototypes in place and are ready to be used in the field immediately (or at least, once the business side of things is figured out).
Will these solve the world’s water crisis? Maybe not by themselves, and maybe not as is. However, they can help provide real-world relief (more than some places are getting) to areas hit the hardest by dwindling drinking water resources. They could provide clean drinking water after a natural disaster, such as a hurricane or earthquake. And they also show where this type of research is heading.
- “The drought in the western U.S. could last until 2030” ↩
- “Renewable Energy Desalination for Island Communities: Status and Future Prospects in Greece” ↩ ↩ ↩ ↩ ↩ ↩ ↩
- “Opportunities and Challenges of Seawater Desalination Technology” ↩ ↩
- “Water Harvesting” ↩
- “From Seawater to Drinking Water at the Push of a Button – With No Filters!” ↩ ↩ ↩
- “A system that’s worth its salt” ↩ ↩ ↩ ↩
- “Hydropanels; a self-sustaining renewable water technology “ ↩
- “Low-Cost Gel Film Can Pluck Drinking Water From Desert Air” ↩ ↩
- “Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid environments” ↩
- “Report Sets Dietary Intake Levels for Water, Salt, and Potassium To Maintain Health and Reduce Chronic Disease Risk” ↩

















Comments