Hydrogen is number one on the periodic table, but it’s still a straggler in the renewable energy race. Like many other renewable technologies, hydrogen has dominated headlines for years now, but inefficiencies in the production and storage of hydrogen hold it back. There’s a lot of pieces to the hydrogen puzzle and one of those pieces has had some recent breakthroughs that may help with those downsides. Is green hydrogen within reach? Let’s see if we can come to a decision on this.
If you’ve been to my channel in the past few years, you’ve probably already heard about hydrogen power before. I tend to talk about it a lot. Partly because, for decades, it failed to materialize as promised, but there is still some hope. I’m fascinated by that dissonance. But for a super quick recap on where things stand:
Hydrogen is LITERALLY all around us. It’s the most plentiful element in the universe, and it’s also one of the most clingy elements out there (meaning it binds to other elements easily, which makes it one of the best energy carriers around). It’s also very clean. How clean? Well, add in a little oxygen in a fuel cell, and hydrogen power leaves behind only water as a byproduct.
You can get hydrogen power from a variety of sources, including fossil fuels and biomass (more on that later!) To get hydrogen alone, because it’s very clingy, you have to break it away from other elements (like the oxygen in water). This takes energy and time, which is the root of hydrogen’s unfortunate energy inefficiency. It takes more energy to produce the hydrogen than you get out of it, and that loss is huge compared to other power technologies like batteries in EVs. You’re losing about 60% from start to finish!1
The way that we get that energy also matters. Hydrogen power has been classified into three categories: gray hydrogen (which gets that energy from fossil fuels), blue (when you combine carbon capture with gray hydrogen practices), and green hydrogen (which comes from renewable energy sources like solar and wind).
Obviously, green is the goal here. Unfortunately, it’s a lot easier in theory than in practice. The two main ways we get green hydrogen are through a renewables-powered electrolyzer (a.k.a. power-to-gas (P2G) technology) and steam reforming of biogas, with or without carbon capture. Most hydrogen power today comes from steam reforming of natural gas, but natural gas is a limited and highly-sought-after resource in itself.2 Using electrolyzers, on the other hand, has been notoriously expensive and inefficient.
So that’s where we last left our discussion on hydrogen power: still struggling with the efficiency, scale, and the overall carbon footprint of this technology in real life. We still need something new and innovative to help us create, store, and transport hydrogen properly. The question is has the efficiency of these systems improved with the latest technological developments?
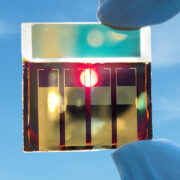
Hysata capillary-fed electrolyzer
Hysta’s new capillary-fed electrolyzer brings a fresh new take to the technology itself.
Polymer electrolyte membrane tech (or PEM, for short) has a problem: as cool as it is, it’s still wildly inefficient. Hydrogen stores more energy per weight than other technologies like batteries, but it also leaks that precious power at every step. You try keeping the smallest element on the planet from literally seeping through the cracks! From its birth in electrolysis to its final resting place in a fuel cell, you’re constantly losing that potential energy. So how can you stop the bleed?
Hysata may have found the culprit and the answer: bubbles. Yes, bubbles. Remember, electrolysis converts water into hydrogen and oxygen. Bubbles in that electrolyte fluid are nonconducting, and when they build up on the electrodes, it is blocking parts of the electrode and hindering the process, reducing the efficiency. PEM lets the cathode side keep running to the electrolyte (which can produce hydrogen gas by bubbling it through a liquid), but Hysata’s capillary-fed electrolyzer takes this a step further. They use a reservoir at the bottom of the cell which keeps the electrolyte away and out of contact with both the anode and the cathode until it’s drawn through an inter-electrode separator (which is both porous and hydrophilic). This is the “capillary action” part: think of it like water rising up a straw due to the change in pressure. It’s relatively simple physics, with some major impacts.
The result: the electrolyte keeps direct contact, but only on one side, and the gasses are still produced without that annoying bubbling action that just gets in the way. Since water isn’t being drawn to the side with the electrode, the other side can keep releasing gas unhindered. As water is electrolyzed out of the separator, the capillary action naturally draws up more water to replace it, keeping everything running smoothly.
So how efficient can these electrolyzers be without those pesky bubbles in the way? According to Hysata’s peer-reviewed study, it can be as much as 98%. That’s over 10% more than a state-of-the-art commercial electrolyzer on the market today, which boasts an efficiency of only 83%.
If these capillary-fed electrolyzers can get into gigawatt-scale production by 2025 (as their CEO claims), it may just fill the need for a cheaper, super-efficient electrolyzer: exactly what hydrogen power needs to really get going.
A new anion-exchange membrane
Of course, Hysata isn’t the only one in the electrolyzer improvement race. A team from the Korean Institute of Science and Technology (KIST) says that it’s tested a new type of membrane that solves the corrosive problem in electrolyzer production. As I mentioned, most electrolyzers use proton exchange membranes (PEMS) which allow positively-charged hydrogen ions to pass through to the cathode and ultimately combine with electrons to form the hydrogen gas. Unfortunately, this acidic environment is super harsh on the equipment, which means these companies need to use expensive metals like platinum, ruthenium, or iridium in their electrodes (as well as titanium on their separator plates).
Also, to speed up the reaction, one of the major expenses is the use of these Platinum Group Metals (PGMs) as a catalyst. These catalysts are extremely expensive and are subject to poisoning by various impurities, such as carbon monoxide and other gasses that may be contaminants in the input gas stream.
The usual solution to this problem is to use anion exchange membranes (AEMs) instead. These membranes and electrode assemblies allow negatively-charged hydroxide ions to pass through and recombine to make water molecules at the anode, ultimately allowing the hydrogen atoms to gravitate towards the cathode. AEMs work under even alkaline conditions, so they generally don’t need the fancy metal catalysts of their PEM counterparts (which can be 3,000 times more expensive). However, AEMs haven’t quite caught their footing in the market, mainly because they generally don’t perform as well and tend to break down more easily.
KIST has recently tested a new membrane and electrode assembly that outperformed their AEM counterparts by a factor of six, and also lasted ten times as long. It even performed 20% better than current PEM technology!3 They did this partially by increasing the specific surface area within the structure and combined this with poly(fluorenyl-co-aryl piperidinium) (or PFAP)-based materials, which have a high ion-conductivity. That’s more surface area, more conductivity, and (in theory) more energy produced over its lifespan, and ultimately, lower costs overall.
How does it hold up?
The hydrogen industry looks like it hasn’t lost any steam. The question is, are these new developments all that they promise to be?
First, let’s talk about efficiency. Hysata’s capillary-fed electrolyzer, for example, only takes 41.5kWh per kilogram of hydrogen to run, compared to 52.5 kWh of current commercial electrolyzers. That’s a whopping 95% overall system efficiency,4 which blows the industry’s standard 75% efficiency out of the water. (And to their credit, they’re not just throwing out this record-breaking claim without any backup: their research was peer-reviewed and published in the March issue of Nature Communications if you’d like to see more.) Their design ensures that you squeeze out every drop of hydrogen that you can, without a single bubble of it going to waste.
On the other side of things, you have developments like the KIST AEM. Their membrane outperforms current PEM technology, but it falls short in terms of longevity. Even though this AEM last six times as long as other AEM counterparts, PEM technology still leaves them both in the dust. PEM electrolyzers typically last around 50,000 hours (or nearly six years), while AEM may only last 3,000 hours.5.
Sure, replacing the KIST AEM may be cheaper, but you’re still replacing them more often, which cuts into your cost savings over time. You also have to consider the versatility. Professor Young Moo for Hanyang University believes this membrane has a crucial role to play not only in electrolysis but also in hydrogen fuel cells, carbon capture utilization, and direct ammonia fuel cells: aka, the next generation of the hydrogen industry.
It’s a tricky balancing act: you want to capture as much hydrogen as possible, without adding in a bunch of extra steps in the process that guzzle time and energy as a result. The more we streamline and perfect this tech, the more the efficiency rate goes up, which helps green hydrogen stack up a little bit better compared to its other renewable counterparts.
Of course, this tiny element that offers so much potential has a problem: costs. Besides energy costs, developing hydrogen infrastructure is expensive. The tech right now takes a lot of precious materials, such as PGMs and rare earth metals, as well as energy to get things going. According to the International Renewable Energy Authority, rare metals factor into the total cost of the PEM system (especially for iridium)3, but at most, they represent about 10% of the total cost. The rest comes from the design itself. The goal is to make hydrogen power cheaper, but will this tech make that a reality?
Unfortunately, that largely still remains to be seen: at least, in the wild. Many of these breakthroughs are still in the early stages. Some are projects in the works or just past their trial stages. They’ll need an infusion of funding and lots of trial and error to get them up to the scale that hydrogen enthusiasts dream about.
Hysata is already planning to have their capillary-fed electrolyzer tech commercialized and up to gigawatt-scale production capacity by 2025. Their tech is meant to cut back on capital and operation costs, too: the gear can be air-cooled, and you don’t need liquid circulation, gas-liquid separator tanks, or any of the related piping, pumps, and fittings. By generating more hydrogen AND reducing capital/operation costs, this tech could potentially drive the cost of green hydrogen down, which they say could bring the cost of hydrogen to $1.50 per kilogram. For reference, right now, you’re looking at about $11 per kilogram … that’s quite the discount! The good news is that green hydrogen’s costs are already on the decline, with renewable and electrolysis costs steadily dropping about 50% every 5-6 years6.
So is hydrogen power finally in its spotlight moment? Well…maybe not quite. A lot of these technologies are still getting their feet wet, and more seem to come out every day; several were announced while writing this script, and if I held off to make sure they all got included, well, this video may never have been finished! (I didn’t even get to talk about how we can turn plastic into hydrogen fuel—now that may be a fun discussion for later!) In the end, these advancements show that this industry is still alive and kicking, which is a great sign as we need a variety of clean energy sources for a cleaner future.
- “Hydrogen Fuel Cell Efficiency: How Does it Compare to Lithium-Ion?” ↩
- NREL – “Hydrogen Basics” ↩
- New Atlas – “‘Game-changing’ anode exchange membrane promises cheaper green hydrogen” ↩ ↩
- New Atlas – “Record-breaking hydrogen electrolyzer claims 95% efficiency” ↩
- “Durability of anion exchange membrane water electrolyzers” ↩
- PV Magazine – “Water-based electrolysis for green hydrogen earns funding” ↩













Comments