Solar and wind power have proven themselves to be cost competitive alternatives to fossil fuels, but to be a truly effective power source alternative, energy storage is key. While lithium batteries opened up the incredible potential for many of the technologies we take for granted today, there still are some issues when trying to scale the technology up for the grid. Expensive casings, overheating, relatively short lifetimes, and battery cell supplies are just a few of the challenges for lithium technology. But what if I told you that molten metal might make a better battery … and no, I’m not talking about the end of Terminator 2 … but we’re talking about lower cost, simpler assembly, zero maintenance, and a longer lifetime than lithium-ion. Let’s take a closer look at liquid metal battery technology.
In a recent study analyzing the effects of the energy transition, the International Renewable Energy Agency (IRENA) found that more than 80% of the world’s electricity could come from renewables by 2050. Solar and wind power could account for 52% of total electricity generation at that point, mainly being driven by declining costs and the spread of green policies. However, their intermittency is sometimes seen as an impediment and a knock against renewable energy, but on the flip side, it’s also pushed interest in energy storage forward like never before. 1
It’s won’t be a surprise when I say this, but the most popular and widespread technology for energy storage is lithium-ion. Shocker. The price of lithium-ion batteries has fallen by about 80% over the past five years, and they’re the reason why electric cars like the newly announced Tesla Model S Plaid can accelerate to 60 miles per hour in as little as 1.99 seconds. That gives a whole new meaning to running to the store to pick up some milk. But lithium-ion batteries aren’t the most practical for storing hundreds of kilowatts or megawatts at a stationary facility. Not to mention, they have a few safety concerns.2 3
The major hazard for lithium-ion battery technology is ‘thermal runaway’ – a cycle in which excessive heat keeps generating more heat. This problem can be caused by internal cell defects, mechanical failure and damage, or overvoltage, leading to high temperatures, gas build-up and potential explosive rupture of the battery cell. Without disconnection, thermal runaway can also spread from one cell to the next, causing further damage. To add to that, a failure in the Battery Management System (BMS) can lead to overcharging and an inability to monitor the temperature or cell voltage. And to wrap up the challenges list, lithium-ion batteries are very sensitive to mechanical damage and electrical surges that can result in internal battery short circuits, leading to all of the previously mentioned issues.4 Sounds like I’m bagging on lithium-ion, which I’m not … they’re great … but they do have challenges.
So when you combine those issues with the still somewhat high cost of lithium-ion of massive battery installations, it shouldn’t be a surprise that there’s still a lot of interest and research into other forms of large scale energy storage technology. One of those is liquid metal batteries … that’s right … molten metal.
And just as a quick refresher for how a typical battery works, they’re composed of an anode (negative electrode), a cathode (positive electrode), a separator between the two electrodes (to keep things from short circuiting), and an electrolyte that fills the remaining space in the battery. The anode and cathode are able to store lithium ions, so as energy is stored during the charge cycle, lithium ions move from the cathode to the anode through the electrolyte. When the battery is discharging, the ions flow back towards the cathode, which feeds the load.5


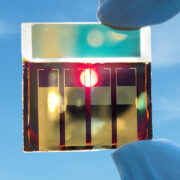
Unlike most batteries, in which the electrodes — and sometimes the electrolyte itself — are solid, in liquid metal batteries all these parts may be in the liquid state. Professor Donald Sadoway at the Massachusetts Institute of Technology pioneered the research of liquid metal rechargeable batteries, using both magnesium–antimony, and more recently lead–antimony. This technology is composed of a metal anode, metal cathode, and salt electrolyte, all in the liquid phase. 6
The anode is a low-density liquid metal that readily gives electrons and the cathode is a high-density metal that’s happy to accept those electrons. The electrodes are coupled through a medium-density molten electrolyte that allows the ions to flow between anode and cathode. 7 8
Imagine you get a bottle and you add a little bit of syrup (or maybe honey), some water, and oil. Once things settle, you’ll notice a segmentation in the mixture in three different layers. This happens because of the different liquid densities, and is exactly what we have in liquid metal batteries. It’s just liquid metals and not delicious syrup. You really don’t want to drink it.
Because of the differences in density and the immiscibility of the three materials, which means they won’t mix together, they naturally settle into three distinct layers and keep separate during operation. The cell is loaded with layers of powdered anode, electrolyte, and cathode materials. When it’s heated to melting, they naturally become three immiscible liquid layers, controlled by their density differences. 7 8
The technology was first proposed in 2009 based on magnesium and antimony separated by a molten salt. Magnesium was chosen for the negative electrode because of its low cost and low solubility in the molten-salt electrolyte. Antimony was selected as the positive electrode because of its low cost and higher discharge voltage. 6
When it comes to operation, liquid metal batteries operate like conventional batteries. During discharging and recharging, positively charged metallic ions travel from one electrode to the other through the electrolyte, and electrons flow to the external circuit to supply the load.
But you kind of have to prime the pump with liquid metal batteries. The initial start-up of liquid-metal requires external heating to melt everything, but once the battery is in a molten state, it maintains operating temperature by generating heat whenever current flows during charge and discharge cycles. It’s designed to be used daily and not to sit idle for weeks at a time. Ideally, we’re talking about a 5 hours charge time, 7 hours idle, 5 hours discharge time, 7 hours idle, that kind of thing every day.7 It’s why this type of battery makes sense for daily renewable energy storage and use.
And I know this topic is going to get a lot of comments about solid state batteries, but there are many pros of liquid metal batteries over solid state. They have a fast electrical response, lower mechanical stresses since the electrodes and electrolytes are liquids, which also means there’s no need for membranes and separators. This all improves long-term stability, usefulness, and provides lower-cost cell fabrication when compared to other conventional batteries. Another reason for the lower manufacturing cost is because the materials used are abundant and cheaper than other materials. But I’ll get to that in a minute. 7 [^solar_feeds 7
Like everything else in the technology world, this isn’t perfect. Liquid metal battery cells are composed of highly corrosive components and have high self-discharge rates for some chemistries due to the metallic solubility of the electrode in the molten salt electrolyte. Bottom line: they tend to not hold onto a charge for long periods. And the biggest elephant in the room, and one you probably thought of during the tasty syrup explanation, the three liquid layers make the battery operation more sensitive to movement and potentially dangerous when the liquid electrodes touch, leading to a short-circuited cell and fleeting heat generation. This isn’t the case for all forms of the battery, which I’ll get to in a moment, but this isn’t something you’d want in your mobile phone … also because it’s insanely hot … but it’s perfect for stationary uses like grid energy storage. 9
Although this technology has many advantages that could make it an ideal solution for grid-scale energy storage, it’s a technology that’s still under development. But when you talk about liquid metal batteries in commercial scale, the Massachusetts-based Ambri is the name that comes up.
In 2010, Donald Sadoway — the pioneer of liquid metal batteries — together with David Bradwell and Luis Ortiz co-founded Ambri with seed money from Bill Gates and the French energy company, Total S.A. They have 50 patents in the US and around the world covering everything from cell chemistry to manufacturing processes. This strong global patent portfolio gives them a massive edge in the marketplace. 10 11
Ambri’s product is a ready-to-install DC containerized system, complete with shelves of cells, weatherproof outer enclosure, thermal management, and a battery management system (BMS). It’s an ideal solution for applications that demand high energy density, frequent cycling, long life and high efficiency.
Cells are assembled onto trays and connected within a thermal enclosure to form a MWh-scale system. The system is insulated and “self-heating”, so it doesn’t require any external heating/cooling to keep the batteries at operating temperature. And the storage capacity can be scaled up by connecting an unlimited number of Ambri systems in parallel.
The current system offered by the company can store from 400 to 1,000 kWh, giving up to 250 kW of power. Ambri’s battery reaches over 80% of efficiency under a wide operation range, similar to lead-acid batteries (like what you have in your car), which achieve efficiencies closer to 80% to 85%, but considerably less than most lithium-ion batteries that see efficiencies higher than 95%.11 12

During transportation, cells are shipped at ambient temperature and are inactive, which provides significant safety advantages during assembly and transportation. And once it’s delivered to the final site, heaters inside the system raise the cells up to operating temperature. Even though the system is expected to remain at operating temperature continuously for its lifecylce, the cells are able to undergo dozens of thermal cycles, from room temperature to 500°C, without impacts on their performance. 11
Unlike lithium batteries, Ambri cells are highly tolerant of over-charging or over-discharging and have a much lower rate of degradation. When a conventional battery is deep cycled (going from 0% to 100% charge) it degrades over time. If lithium-ion batteries are subjected to a daily deep cycle like that, they can lose 20% of their capacity in just 2 years. On the other hand, the Ambri battery can be deep cycled every day for 20 years and lose as little as 5-10% of its capacity. In energy facilities, deep cycles occur frequently since the batteries are regularly charged and discharged to make up for the fluctuations in the power grid.
13
Ambri’s liquid metal battery is made of a liquid calcium alloy anode, a molten salt electrolyte and a cathode comprised of solid particles of antimony, enabling the use of low-cost materials and a low number of steps in the cell assembly process.


Ambri is starting with initial demonstration systems. By 2022, they want to have a 1 MWh commercial system developed, certified and deployed for trials, and in 2023 their target is 250 MWh shipped for commercial projects. 14
Kicking things off, they recently signed an agreement with TerraScale. As part of the agreement, TerraScale and its data center development partners will integrate an Ambri energy storage system for its Energos Reno project near Reno, Nevada. There’s already 10MW of solar generation built at the site, which TerraScale intends to bring up to 500MW and 23MW of active geothermal power with a rated capacity of 48MW. 15 16
Adam Briggs, Ambri’s Chief Commercial Officer said:
“The collaboration is underway and includes delivery of 250 MWh of Ambri systems to TerraScale’s first project in Reno, Nevada starting in 2021.”
“The Ambri systems are particularly well suited for the project’s high-desert operations, for the shifting of its large amounts of renewable solar load, and for its grid-system peak shaving capability.” 15
With all of the liquid metal advantages I ran through, there are challenges since most current technologies operate at temperatures above 240°C to keep metallic electrodes in a molten/liquid state, which can obviously pose serious problems. In addition to the complex thermal management there’s also the concern about corrosion.
Professor Guihua Yu and his team from the University of Texas at Austin carried out a study on room‐temperature liquid metal battery that used a sodium-potassium alloy anode and gallium-based alloy cathode. According to the researchers, the metallic electrodes in the team’s battery remained liquefied at 20°C (68 °F), the lowest operating temperature ever recorded for a liquid metal battery.
The researchers have been working on this project for three years, but the work isn’t complete yet. Finding an alternative to the expensive gallium that can provide the same performance remains a major challenge. 17
Overall, the cycling stability, the requirement for less maintenance, and the lower cost and availability for manufacturing provide a lot of room for liquid metal batteries to catch on for grid energy storage. It’s expected to reach a market value of $6.7B by 2027.18 It’s always important to remember that there’s no one technology to rule them all. Having more options like this can only help with our need to stabilize energy generation from cheap, intermittent renewables like solar and wind.
- Electricity storage and renewables: Costs and markets to 2030 ↩︎
- Declining Renewable Costs Drive Focus on Energy Storage ↩︎
- Model S ↩︎
- Lithium-ion Battery Energy Storage Systems – The risks and how to manage them ↩︎
- How lithium-ion batteries works? ↩︎
- Molten-salt battery ↩︎
- Getting a charge out of liquid metal batteries ↩︎
- A battery made of molten metals ↩︎
- Liquid Metal Battery ↩︎
- Ambri – Business ↩︎
- The Ambri technology ↩︎
- Lithium-ion vs. lead acid batteries ↩︎
- The next step in renewable energy? Molten metal batteries ↩︎
- Ambri – Benefits ↩︎
- TerraScale’s Energos Reno Project releases plans to develop a data center with 500MW of renewable power ↩︎
- Ambri’s liquid metal battery to be used at desert data centre in Nevada ↩︎
- A non-toxic, room-temperature liquid-metal battery ↩︎
- Credence Research – Next Generation Luquid Metal Battery Market ↩︎













Comments