Imagine a future where every home, office or building is painted with solar panels and its bricks operate as batteries thanks to nanotechnology. There’s a lot of promise, but what is nanotechnology? And is it more science fiction than fact?
When you hear the term nanotech, chances are some sci-fi book or movie pops into your head, where they used the term to explain away some technological wonder or advancement. “Don’t worry about that, it’s nanotech!” It’s become a deus ex machina for science fiction writers.
But what we’re starting to see is that nanotechnology is responsible for great advances in physics, biology, chemistry, engineering and material science. It’s responsible for the new age of modern technology that will help civilization reach for the stars and more.
Nanotechnology refers to our ability to study and engineer technologies at a nanoscale, which is the range from 1 to 100 nanometers. That begs the question, “how small is a nanometer?” Well, if I tell you “A nanometer is one billionth of a meter … or one millionth of a millimeter” I don’t think that really clears things up. I don’t know about you, but my brain breaks trying to think about that scale. So, let’s try to put it in perspective: a human hair is around 75,000 nanometers wide – and remember, the range for nanoscale is 1 to 100 nanometers. Still not doing it for you? Let’s flip it around. Imagine a marble measures 1 nanometer. In comparison to that, the Earth would measure about one meter in diameter.1 Let that sink in for a minute… a marble compared to the size of our entire planet … that’s 1 nanometer compared to 1 meter.
Given how mind-boggling these scales are, we definitely have to give credit to the father of nanotechnology, Physicist Richard Feynman. It all started with the American Physical Society meeting held at the California Institute of Technology on December 29, 1959. Feynman gave a talk titled “There’s Plenty of Room at the Bottom,” where he speculated about being able to construct machines down to the molecular level — and the concept behind nanotechnology was born. It wasn’t until 1974 that the term “nanotechnology” was coined by Professor Norio Taniguchi, while he worked on ultraprecision machining. As he put it: “nanotechnology mainly consists of the processing of separation, consolidation, and deformation of materials by one atom or one molecule.” We had the concept, then the term, but it wasn’t until 1981 that this theory became a reality with the development of a scanning tunneling microscope that helped scientist actually see atoms individually. Gerd Binnig and Heinrich Rohrer developed the microscope at IBM Zurich Research Laboratories in Switzerland and were later awarded the Nobel Prize in physics in 1986. That major achievement was followed by the Atomic Force Microscope in 1985, which had the distinct advantage of imaging on almost all surfaces, including biological samples, glass, composites, and ceramics. This would prove to be a major turning point.
With the advent of nanotechnology, scientists were now able to manipulate individual atoms. And that takes us into the realm of quantum mechanics, which is the science behind how matter behaves in atomic and subatomic scale. Thankfully, that’s out of scope for this video since that breaks my brain even more, but basically materials at this scale tend to behave differently and exhibit distinctive chemical and physical properties. Scientists were keen to learn and exploit this attribute to craft materials at nanoscale.
Since 1981, we’ve come forward leaps and bounds in the field of Nanotech. There’s so much that I could cover, but in the interest of time, I’ve picked two categories of examples that are helping to make what seemed like science fiction into science fact for our future. But I’d love to hear in the comments if there are any topics or examples you’d like to see covered in a future video.
Solar
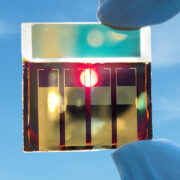
The first category is one that I talk about a lot: solar. Nanotechnology is leading the charge for solar energy. Most silicon based solar panels, which accounts for about 95% of commercial solar, utilize nanoscale processes for manufacturing. Some are multi-junction solar cells, which layer different solar technologies to broaden the wavelengths of light that are captured and converted into energy. This layer cake of solar cell technologies are measured in nanometers. Thinner than a width of a human hair. But it’s the next generation of solar cells that are being researched now that could takes things to a whole new level.
Solar-Collecting Paint is an exciting future possibility.
Imagine the paint on your house or a building acting as a solar panel? Or how about your car? Chemistry professor Richard L. Brutchey from University of Southern California and researcher David H. Webber successfully developed solar collecting paint by using solar-collecting nanocrystals. At only 4 nanometers in size, nanocrystals can float in a liquid solution. You could potentially fit 250 billion nanocrystals on the head of a pin, they’re THAT small. Brutchey and Webber were able to find an organic molecule that would keep the nanocrystals conductive without sticking to each other.
So why isn’t this available in the market yet? Well those nanocrystals were built with cadmium, which is a toxic metal. Researchers have been busy trying to find alternative materials and there are some really promising leads.
Quantum dot solar cells
Quantum dot solar cells are one area to look at. Quantum dots are semiconducting particles that behave differently due to their size and the effects of quantum mechanics, like I mentioned earlier. They have energy similarities to atoms, which is why they’re sometimes referred to as “artificial atoms.” In June 2020 researchers at the Los Alamos National Laboratory were able to create cadmium-free Quantum Dot solar cells. Their zinc-doped variant has a high defect tolerance and is toxic-element-free.
This year researchers at the University of Queensland were even able to break a new world efficiency record of 16.6% for a quantum dot solar cell made from a halide Perovskite. That’s a 25% improvement in relative efficiency compared to the last record holder from 2017, so there’s fast progress being made. But the big challenge is around commercialization of the breakthrough, so the university is working on a large scale printing process in addition to continuing to improve the efficiency.
Perovskite solar paint
In 2014, researchers at the University of Sheffield were able to develop a spray on solar cell using Perovskite which is a class of man-made compounds that share the same crystalline structure as the calcium titanium oxide mineral with the same name.2,3 It happens to be one of the most promising solar technologies in recent years because it has a broad absorption spectrum. It consists of a 300 nanometer thin film with a crystal structure that aids solar absorption and can operate efficiently during cloudy days as well. Scientific Director at Saule Technologies, Dr. Konrad Wojciechowski, says that this could be printed using an inkjet printer.4
Swedish firm Skanska tested it on a building in 2019 and is expected to start producing it in 2021 with the expected cost to be $58 per meter and an efficiency around 10%.
The reason why all of these examples are so exciting is that a paintable solar cell opens up the floodgates for where you can apply solar power. Painting the walls of a building, not just the roof, or as I mentioned earlier, your car. It should also help to reduce the costs of manufacturing solar technologies, which will make it more accessible. It’s potentially a huge win/win.
Energy Storage
The second category I wanted to look at for this video is nanotechnology being applied to energy storage. In a previous video I’ve walked through graphene and carbon nanotubes and how they’re impacting energy storage today. Specifically, in my supercapacitor video I talked about how companies like NAWA Technologies and Skeleton are building out graphene-based supercapacitors today. Skeleton’s products can be found helping to power major tram-systems in big European hubs like Warsaw and Mannheim.5
As a quick refresher, batteries and supercapacitors share some similarities in how they work. In a battery there’s a positive and negative side, which are called the cathode and anode. Those two sides are submerged in a liquid electrolyte and are separated by a micro perforated separator, which only allows ions to pass through. When the battery charges and discharges, the ions flow back and forth between the cathode and anode. But capacitors are different, they don’t rely on chemical play in order to function. Instead, they store potential energy electrostatically. Capacitors use a dielectric, or insulator, between their plates to separate the collection of positive and negative charges building on each plate. It’s this separation that allows the device to store energy and quickly release it6. It’s basically capturing static electricity.
In one recent advancement in batteries from July 2020, scientists from Clemson Nanomaterials Institute were able to achieve high rate capability, fast diffusion, high capacity, and a long cycle life thanks to sandwiching silicone nanoparticles with carbon nanotubes called bucky papers.7 The cycle life for lithium batteries with silicon based anodes is less than 100, but thanks to the new sandwiched silicon electrode structure they were able achieve 500 cycles and deliver three times more capacity than graphite. Silicone happens to have ten times higher capacity than graphite, but it expands by about 300 percent in volume as it absorbs ions. The end result is an anode that breaks apart. This nanostructure counters this factor and would help us replace graphite with silicone, so that our batteries can become safer and lighter.
But I’ve saved the craziest research I’ve seen in a while for last… Nanotechnology could potentially turn bricks into batteries. …well, more like supercapacitors, but that doesn’t have the same alliteration. Washington University’s Institute of Materials Science & Engineering took work from their microsupercapacitor research using Fe2O3 (iron oxide – or rust) as a conducting polymer, also known as rust-assisted vapor-phase polymerization. Rolls right off the tongue. I’m not going to get bogged down into the technical details, partially because of my broken brain, but what sets this process apart is that the nanostructures formed by this process are self-assembled. Other processes like this might take several steps and treatments, which makes this process unique.
So I can hear you asking how does this possibility relate to bricks? That red pigment in your classic brick is … you probably guessed it … Fe2O3 (iron oxide – or rust). By applying their polymer process to a standard red brick, you end up with a capacitor.8 Julio D’Arcy, assistant professor of chemistry, who worked on this research, described it:
“In this work, we have developed a coating of the conducting polymer PEDOT, which is comprised of nanofibers that penetrate the inner porous network of a brick; a polymer coating remains trapped in a brick and serves as an ion sponge that stores and conducts electricity.” -Julio D’Arcy, Assistant Professor9
This process leaves a blue PEDOT coating on one side of the brick, so that could be easily hidden on one side of the brick wall. They estimate that it would take about 50 bricks to power an emergency lighting system for 5 hours, so this clearly isn’t going to power your entire house. But then again, a building is made up of thousands of bricks, so there’s a potential for a building’s brick walls to act as a massive supercapacitor to absorb solar panel overproduction, or to cover peak energy use to smooth out demand, and pair with battery storage in a hybrid setup.
We’re already seeing some of nanotechnologies benefits in the world around us today, but the research and advancements we’re seeing in the lab, like these, are what to look forward to for the future. Nanotech may have been an overused and blanket term that’s lost a little bit of it’s meaning to most of us, but there’s real progress being made.
- Nano.gov, “Size of the Nanoscale” ↩︎
- Energysage, “Perovskite solar cells: the future of solar?” ↩︎
- Wikipedia, “Perovskite solar cell” ↩︎
- Energy & Environmental Science, “Towards the commercialization of colloidal quantum dot solar cells: perspectives on device structures and manufacturing” ↩︎
- Railway Technology, “Skeleton Technologies to provide ultracapacitor for Warsaw tram system” ↩︎
- Green Techee, “How does an ultracap work?” ↩︎
- New Atlas, “Silicon ‘sandwiches’ make for lightweight, high-capacity batteries” ↩︎
- Nature Communications, 11, “Energy storing bricks for stationary PEDOT supercapacitors” ↩︎
- Washington University in St. Louis – The Source, “Storing energy in red bricks” ↩︎













Comments